Nebula Traceability Platform
The nebula traceability platform consists of the IoT device – Shadow-X and the cloud application WMS. It’s designed to monitor cold-chain logistics including the transportation of pharmaceutical drugs and vaccines, medical equipment, reagents, blood products, fresh food, the results of clinical research, and more. With an easy, two-step process, Nebula can be programmed to track the smallest units throught the entire logistics process.
The solution monitors transportation by land, sea and air, as well as cross-border transportation and last-mile delivery.

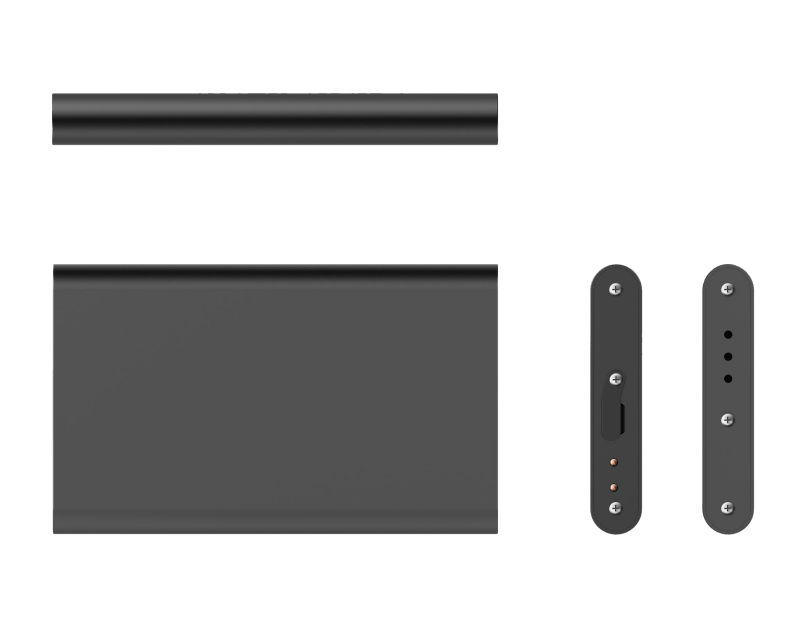
Shadow-X Product Features
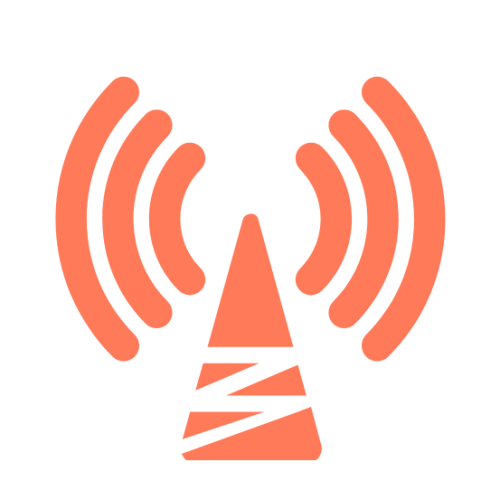
GPS and Global 4G
4G LTE Cat4 communication module, GNSS multi-constellation positioning module (supports GPS, Compass, Galileo, GLONASS)
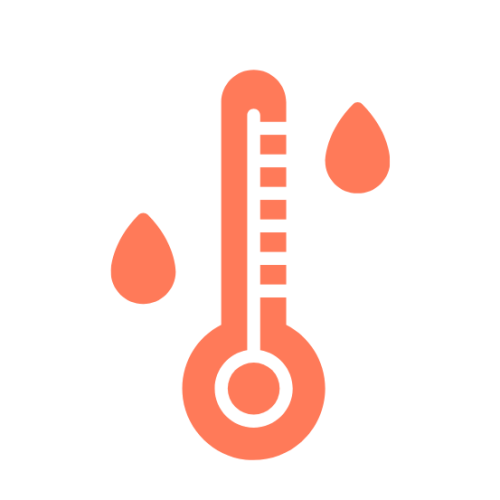
Multi-parameter Monitoring
Swiss high precision temperature and humidity sensors, acceleration sensors, gas sensors, light sensors

IP67 Water Proof
Equipment is IP67 rated and can operate under water for up to 30 minutes
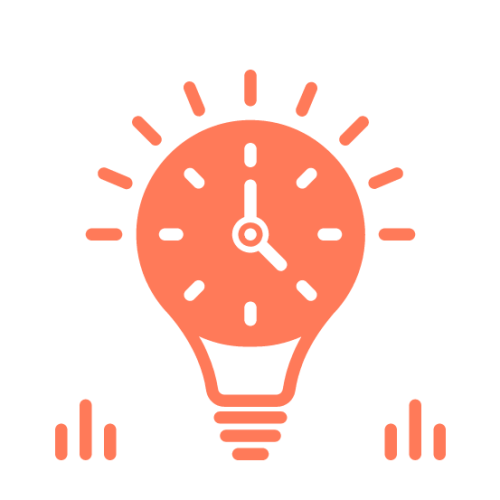
Long Battery Life
Up to 20 days of battery life (default reporting frequency of 15 minutes)

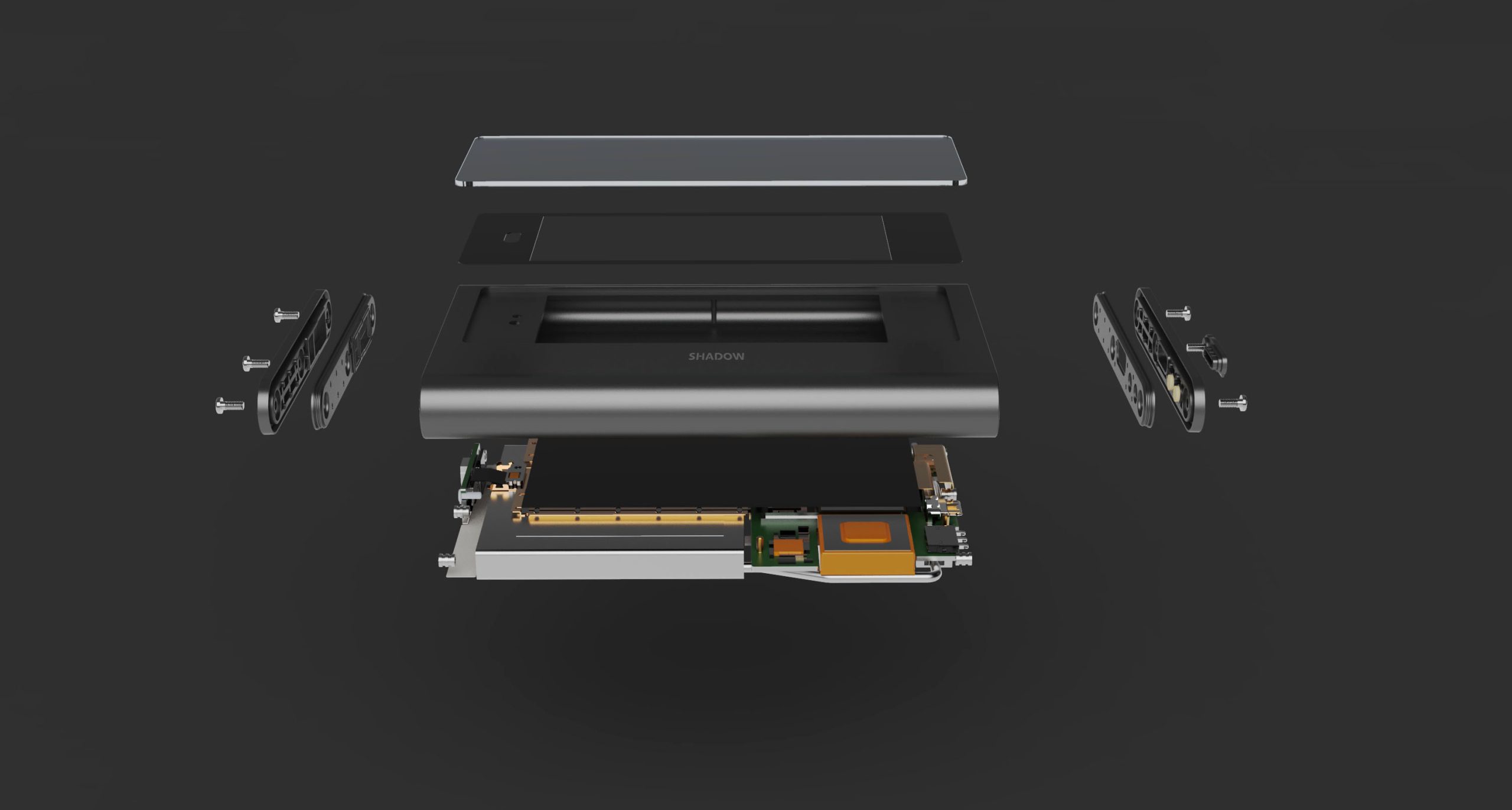
Shadow-X Product Specs
| Temp measurement range | -30℃ ~ +70℃ |
| Temp measurement accuracy | +/-0.5℃ |
| Temp display resolution | 0.1℃ |
| Humidity measurement range | 0 ~ 100%RH |
| Humidity measurement accuracy | +/-5%RH |
| Humidity display resolution | 0.1%RH |
| Device Built-in Storage | >20000 records |
| Light sensitivity range | 0 ~ 128kLux |
| Operating temperature | -30℃ ~ +70℃ |
| Duration of continuous operation | 20 days |
| Water proof class | IP67 |
| Traceability track deviation | <=10m |
The Nebula traceability tracking system has helped customers to
Our products are suitable for customers in a wide range of industries
Medical devices
The highly sensitive requirements of medical devices are often susceptible to impact or misplacement, and current device regulations (MDR), require that product quality be ensured throughout the supply chain
Clinical research
Costly clinical trials that require seamless monitoring of the entire transport process from kit packaging to patient and the ability to make immediate adjustments to deviations
Pharmaceutical vaccines
Due to the complexity of global pharmaceutical cold chain logistics and the stringent FDA regulations for pharmaceutical vaccines, more than 20% of pharmaceutical vaccine products fail and deteriorate each year due to improper temperature control

Fresh food
The cold chain transportation of perishable fresh food is increasingly subject to strict regulations, such as PCR regulatory requirements, which also create greater challenges for the monitoring of the fresh cold chain




